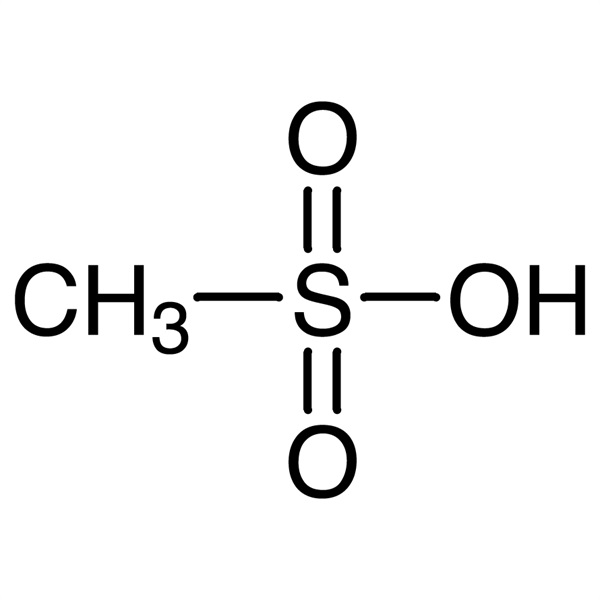
Methanesulfonic Acid (MSA) CAS 75-75-2 Purity >99.5% (T) Factory Hot Selling
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Methanesulfonic Acid (CAS: 75-75-2) with high quality, commercial production. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Methanesulfonic Acid |
| Synonyms | Methylsulfonic Acid; Sulfomethane; Methanesulfonic Acid Sodium Salt; MSA |
| CAS Number | 75-75-2 |
| CAT Number | RF-PI2044 |
| Stock Status | In Stock, Production Capacity 3000MT/Year |
| Molecular Formula | CH4O3S |
| Molecular Weight | 96.11 |
| Sensitivity | Light, Heat and Moisture Sensitive |
| Solubility in Water | Completely Miscible With Water |
| Solubility | Soluble in Alcohol, Ether; Slightly Soluble in Benzene; Very Slightly Soluble in Toluene |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Colorless or Slightly Brown Oily Liquid |
| Purity / Analysis Method | >99.5% (Neutralization Titration) |
| Melting Point | 15.0~20.0℃ |
| Chloride (Cl-) | ≤20mg/kg |
| Sulfate (SO42-) | ≤50mg/kg |
| Iron (Fe) | ≤5mg/kg |
| Heavy Metals (Pb) | ≤5mg/kg |
| Calcium (Ca) | ≤3mg/kg |
| Sodium (Na) | ≤3mg/kg |
| Oxidizabes | ≤30mg/kg |
| Chroma | ≤20 hazen |
| Refractive Index n20/D | 1.4285~1.4315 |
| Specific Gravity (20/20℃) | 1.481~1.486 |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
| Note | This product is low melting point solid,may change state in different environments (solid, liquid or semi-solid) |
| Usage | Pharmaceutical Intermediates |
Package: Fluorinated Bottle, 25kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Methanesulfonic Acid (MSA) (CAS: 75-75-2) is a strong organic acid. It is considered a green acid as it is less toxic and corrosive in comparison to mineral acids. The aqueous MSA solution has been considered a model electrolyte for electrochemical processes. Methanesulfonic Acid is an alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is methyl. It has a role as an Escherichia coli metabolite. It is an alkanesulfonic acid and a one-carbon compound. It is a conjugate acid of a methanesulfonate. Methanesulfonic Acid, the simplest alkanesulfonic acid. It will not subject to decomposition in boiling water and hot alkaline solution. It also has strong corrosion effect against the metal iron, copper and lead. Methanesulfonic Acid is a raw material for medicine and pesticide. It can also be used as dehydrating agent, curing accelerator for coating, treating agent for fiber, solvent, catalysis, and esterification as well as polymerization reaction. It can be used as solvent, alkylation, catalyst of esterification and polymerization, also used in medicine and electroplating industry. It can also be applied to oxidation. Methanesulfonic Acid is used in the electroplating industry and for organic syntheses, in particular as a catalyst for alkylations, esterifications, and polymerizations. Beyond that, Methanesulfonic Acid is used as a starting material for the preparation of methanesulfonyl chloride. Methanesulfonic Acid has been developed as an esterification catalyst in place of sulfuric acid for the synthesis of resins in paints and coatings. One of the major advantages of Methanesulfonic Acid over sulfuric acid is that it is not an oxidizing species. Methanesulfonic Acid is used as a catalyst in organic reactions namely esterification, alkylation and condensation reactions due to its non- volatile nature and solubility in organic solvents. It is also involved in the production of starch esters, wax oxidate esters, benzoic acid esters, phenolic esters, or alkyl esters. It reacts with sodium borohydride in presence of polar solvent tetrahydrofuran to prepare borane-tetrahydrofuran complex. It finds application in batteries, because of its purity and chloride absence. In pharmaceutical industry, it is used for the manufacturing of active pharmaceutical ingredients like telmisartan and eprosartan. It is useful in ion chromatography and is a source of carbon and energy for some gram-negative methylotropic bacteria.It is involved in the deprotection of peptides.
-
Methanesulfonic Acid (MSA) CAS 75-75-2 Purity >...
-
Isopropyl Methanesulfonate CAS 926-06-7 Purity ...
-
Methylene Methanedisulfonate (MMDS) CAS 99591-7...
-
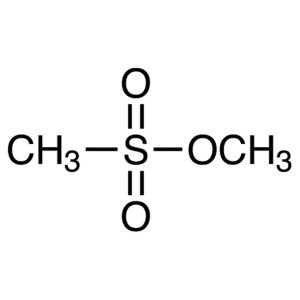
Methyl Methanesulfonate (MMS) CAS 66-27-3 Purit...
-
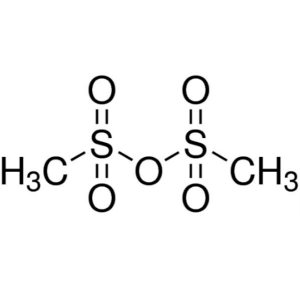
Methanesulfonic Anhydride CAS 7143-01-3 Purity ...
-
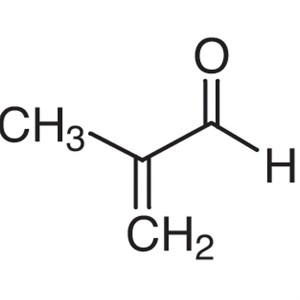
Methacrolein CAS 78-85-3 (Stabilized with HQ) P...
-
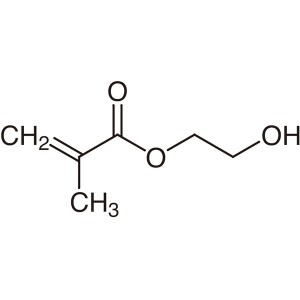
2-Hydroxyethyl Methacrylate HEMA CAS 868-77-9 P...
-
Isopropenyl Acetate (IPA) CAS 108-22-5 Purity ≥...
-
Methyl Isobutyl Carbinol (MIBC) CAS 108-11-2 Pu...
-

Malononitrile CAS 109-77-3 Purity >99.0% (GC) F...
-
Ethyl Formate CAS 109-94-4 Purity >99.0% (GC) F...
-
Ammonium Formate CAS 540-69-2 Purity ≥99.99% (M...
-
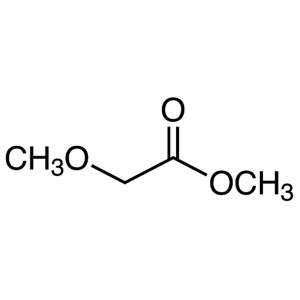
Methyl Methoxyacetate CAS 6290-49-9 Purity >99....
-
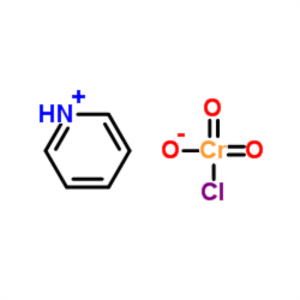
PCC Pyridinium Chlorochromate CAS 26299-14-9 As...
-
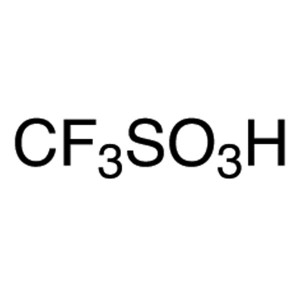
Trifluoromethanesulfonic Acid CAS 1493-13-6 Pur...
-
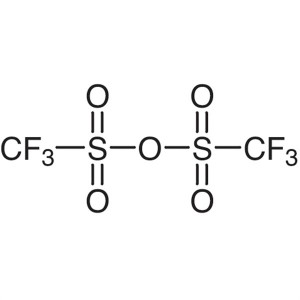
Trifluoromethanesulfonic Anhydride CAS 358-23-6...